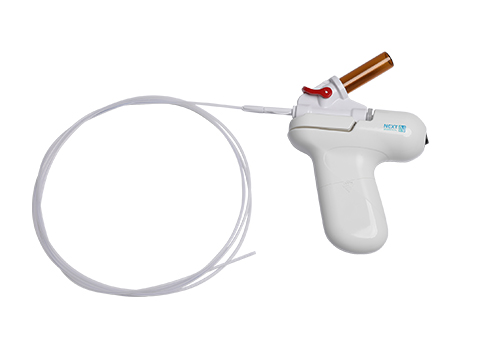
Innovative Hemostatic Systems for Endoscopy
Just Spray It ! Endoscopic hemostatic system for GI bleeding
ContactNexpowder is a powdery wound dressing for endoscopies made of biocompatible polymer, used for gastrointestinal bleeding.
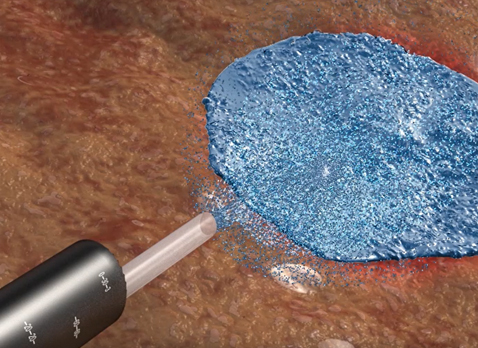
It can be used where the bleed is occurring without direct contact to the tissue, regardless of the bleeding location.
Nexpowder is sprayed through the endoscope, and when it comes in contact with water, as well as blood, gelation occurs; physically applying pressure
to the wound to prevent bleeding.
This adhesive gel also protects the wound, prevents contamination, and loss of body fluids that may occur through the wound.
The gel removes itself and is excreted within 1 to 3 days.
Indication
Nexpowder is a product used for wounds and bleeding sites during hemorrhagic digestive ulcers,
endoscopic mucosal resection (EMR), and endoscopic submucosal dissection (ESD) procedures.
Nexpowder prevents contamination of the affected area, protects wounds, and emits a hemostatic effect.
How to use
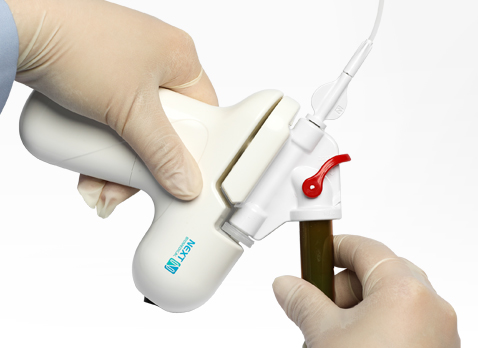
Best practice
Make sure the valve is closed after connecting the catheter to spray body.
Be sure to turn on the power before inserting the catheter into the endoscope channel.
Do not place the catheter directly in contact with the bleeding site.
Do not aspirate while catheter is in the accessory channel.
Features
Immediate
hemostasis rate

The adhesive
Force of other
Hemostatic sprays


Rebleeding
rate

Rebleeding
rate

Global partnership
Ordering information
* 1 procedure kit
| Product Name | Reference No. | Specifications | Quantity |
|---|---|---|---|
| Nexpowder | UI-EWD3 | 3g | 1ea |
| Catheter | EU-2522 SG-2522 |
2.5mm/220cm | 1ea |
| Spray body | UIPSD04 | 1ea | 1ea |