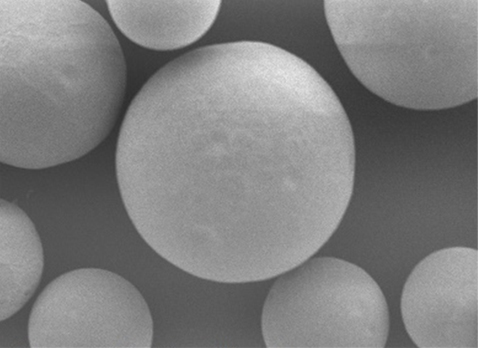
Resorbable Gelatin
Microspheres for
Vascular Embolization
Nexsphere™ is a gelatin based hydrophilic microspheres for endovascular embolizations, that is used together with a contrasting agent during vascular embolization for therapeutic and treatment purposes. It is injected into the blood vessel through a microcatheter in order to temporarily occlude the blood vessel. Nexsphere™ is an resorbable microsphere that exhibits embolic performance in a variety of indications with uniform spherical particles, high elasticity and strong cohesion.
OverviewIndication
Use in embolization of blood vessels to occlude blood flow for therapeutic purposes
Indication

Liver Cancer

Uterine Fibroids

Prostatic Hyperplasis

Osteoarthritis

Arterial Bleeding
Features

Uniform Spherical Particles

Minimization of side effects
without crosslinking agents

Injection container that prevents contamination
Vial designed for contamination prevetion (Design Patent : 3009879200000)

Various size selections depending
on the patient condition
How to use

Inject saline into the Nexsphere™ vial using a luer lock syringe and then shake to disperse and hydrate the microspheres

Slowly inject the contrast medium into Nexsphere™ vial with saline using a luer lock syringe, and shake for minutes to mix the contents.

Transfer the mixed contents to a syringe.

Inject the mixed Nexshpere™ contents into the lesion via a stanard catheter
Best practice
It is recommended to wait for hydration depending on sizes after adding saline to the Nexsphere™ vial and shaking.
Inject contrast medium into the vial containing saline and Nexsphere™
Product highlights
-
Gelatin based resorbable microspheres capable of temporary embolization

Nexsphere

Gelatin sponge
-
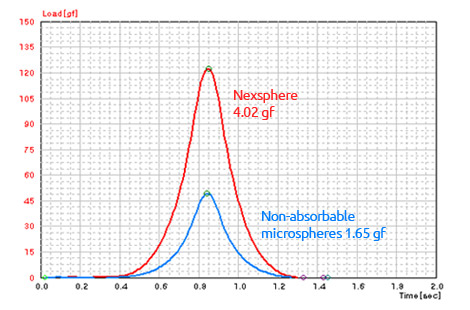
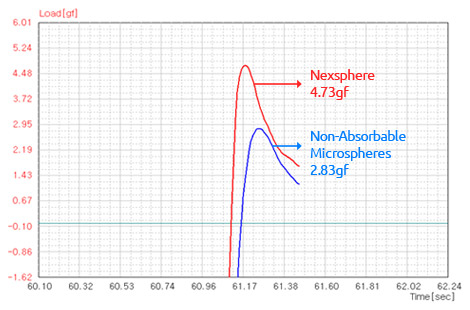
High elasticity and cohesive force1)
-
Absorbable within 2 hours after embolization 2)

Histology of Rat Liver 3)
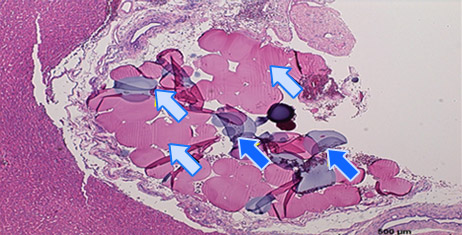
Embolization
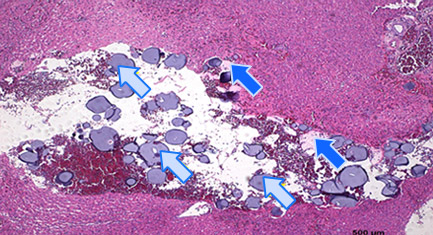
2 hours after embolization
 Non-resorbable product
Non-resorbable product Non-resorbable product
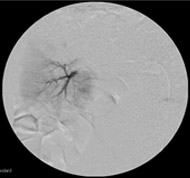
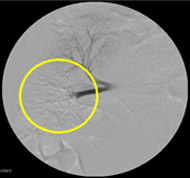
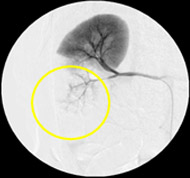
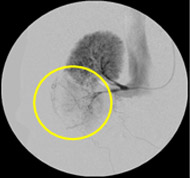
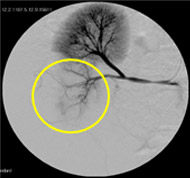
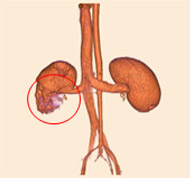
Non-resorbable productAngiography of Porcine Kidney 3)
Pre-embolization
Embolization
Recanalization 2 hours after embolization
-
Effective and safe way to relieve pain and promote functional recovery in patient 4)
No adverse events including skin discoloration 4)
Post-marketing clinical trials in progress
- - Seoul Asan Medical Center in Korea : A prospective, comparative study is currently ongoing to evaluate safety and efficacy or Nexsphere with different absorbable times for transcatheter arterial chemoembolization for hepatocellular carcinoma. (150 patients)
- - Severance Hospital in Korea : Comparison of pain after uterine artery embolization using spherical gelfoam or tris-acryl gelatin microsphere in patients with symptomatic fibroids: A Prospective, Randomized Study. (60 patients)
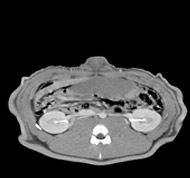
Preclinical outcomes
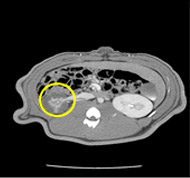
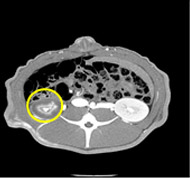
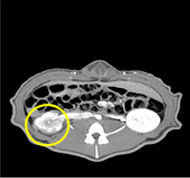
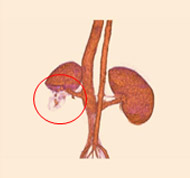
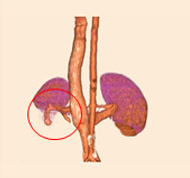
porcine kidney embolization 5)
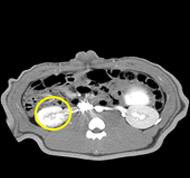
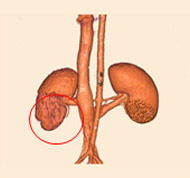
Before
embolization5mins after
embolization1week after
embolization2week after
embolization4week after
embolization
Ordering information
| Ordering Code | Size (㎛) |
Quantity (㎎) |
|---|---|---|
| UIGB 100 | 100-300 | 300 |
| UIGB 300 | 300-500 | 300 |
| UIGB 500 | 500-700 | 300 |
| UIGB 700 | 700-900 | 300 |
| Nexsphere-H | 100-300 | 250 |
| Nexsphere-F | 100-300 | 200 |
References
-
- 1) In-house elasticity and cohesion tests were performed on monolayers of microspheres with a texture analyzer.
- 2) In house in-vitro degradation test showed that Nexsphere-F was degraded in 37°C of warm saline within 2 hours.
- 3) Animal studies were conducted with Nexsphere-F by external non-clinical CRO in Korea. Animal study results may or may not be indicative of clinical outcomes in humans.
- 4) Jae Hwan Lee et al. Short-term Results of Transcatheter Arterial Embolization for Chronic Medical Epicondylitis Refractory to Conservative Treatment : A Single-Center Retrospective Cohort Study. Cardiovasc Intervent Radiol. 2021 June 4.
- 5) Animal studies were conducted with Nexsphere by external non-clinical CRO in Korea. Animal study results may or may not be indicative of clinical outcomes in humans.