Nexsphere-F is a fast resorbable hydrophilic gelatin-based embolic microsphere for endovascular embolization.
It can reduce pain of the musculoskeletal disorder such as arthritis, including knee, elbow, frozen shoulder, etc.
Nexsphere-F is the first approval product in EU that can be used for arthritis as a fast resorbable microsphere.
Mechanism of Degenerative Arthritis

The arthritis advanced by vicious cycle of inflammation, angiogenesis and joint degeneration.
MSK(Musculoskeletal) Embolization

MSK Embolization is a new concept procedure that can be performed for chronic pain in the musculoskeletal disorder.
New blood vessel formation promotes the formation of nerve cells and caused joint pain.
By blocking the abnormal vessel through embolization, the nerve cells are necrosis and pain is reduced or disappeared.
MSK Embolization can be performed on almost joints, such as knee, frozen shoulder, elbow, wrists, ankle, etc.,
and is more effective than conventional treatments because it is a minimal-invasive procedure.
Indication
MSK embolization requires cell necrosis, it is very important to temporarily embolization only a short period of time.




Features

Uniform spherical particles

Minimization of side effects
without crosslinking agents

Vial designed for contamination prevetion (Design Patent : 3009879200000)
How to use

Inject saline into the Nexsphere-F™ vial using a luer lock syringe and then shake to disperse and hydrate the microspheres.

Slowly inject the contrast medium into Nexsphere-F™ vial with saline using a luer lock syringe, and shake for minutes to mix the contents.

Transfer the mixed contents to a syringe.

Inject the mixed Nexshpere-F™ contents into the lesion via a standard catheter.
Best practice
It is recommended to wait for hydration depending on sizes after adding saline to the Nexsphere-F™ vial and shaking.
Inject contrast medium into the vial containing saline and Nexsphere-F™
Product Highlight
-
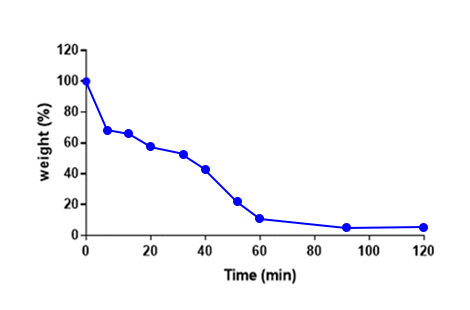
- In vitro absorption test : 1h
- Degradability test1)
-
Effective and safe way to relieve pain and promote functional recovery in patient 4)
No adverse events including skin discoloration 4)
Post-marketing clinical trials in progress
Seoul National University Bundang Hospital : Compatibility and safety evaluation using a preclinical model
Completed a pilot clinical trial in Japan
Angiography of porcine kidney 2)
Pre-embolization
Embolization
Recanalization 2 hours after embolization
Preclinical Outcomes
In vivo test of porcine kidney and liver embolization2)
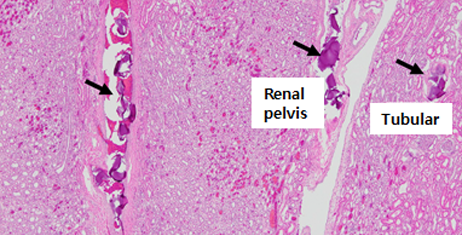
HE staining image of porcine kidney 2 hours after embolization
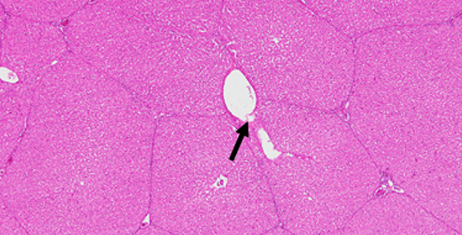
HE staining image of porcine liver 2 hours after embolization
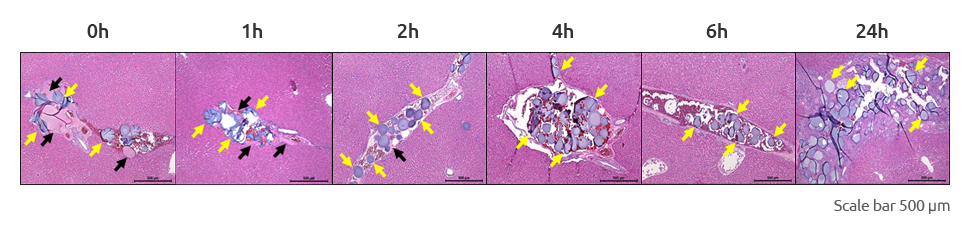
In vivo test of Nexsphere-F™ in rat liver3)
References
-
- 1) In-house test for measuring the weight after removing the vial supernatant and drying the remaining microspheres
- 2) Animal studies were conducted with Nexsphere-F by external non-clinical CRO in Korea. Animal study results may or may not be indicative of clinical outcomes in humans.
- 3) In house in-vivo degradation test showed that Nexsphere-F was degraded within 2 hours.
- 4) Jae Hwan Lee et al. Short-term Results of Transcatheter Arterial Embolization for Chronic Medical Epicondylitis Refractory to Conservative Treatment : A Single-Center Retrospective Cohort Study. Cardiovasc InterventRadiol. 2021 June 4.



